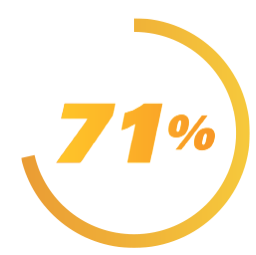
of adults experienced
MOVEMENT
REDUCTION
with AUSTEDO2,3†
†People taking AUSTEDO had at least a 10% reduction based
on the total motor AIMS score at Week 12.3
Once-daily AUSTEDO XR contains the same active ingredient as twice-daily AUSTEDO® (deutetrabenazine) tablets. Data on this page is based on twice-daily dosing.1
>2x reduction
*AUSTEDO reduced the Abnormal Involuntary Movement Scale (AIMS) total score by 3.3 points vs 1.4 points with placebo. For people taking AUSTEDO, this represents a 33% reduction in AIMS total score vs a 14% reduction for those taking placebo.1

MOVEMENT
REDUCTION
†People taking AUSTEDO had at least a 10% reduction based
on the total motor AIMS score at Week 12.3
2 WEEKS1,2
‡This was an open-label study, which is a drug trial in which all participants are aware of the drug being tested.
If you’re ready to learn more about TD, what causes it,
and its impact, this is the place to start.
Get patient support, treatment information, and downloadable
resources to stay on track with AUSTEDO XR.
This Appointment Preparation Guide can help you get the conversation
going with
your doctor about starting AUSTEDO XR.
Austedo XR can be taken with most mental health medications1,3
If you've taken the important step of treating your mental health, treating TD does not have to hinder your progress.
AUSTEDO XR can be taken with most mental health medications—so you can treat your TD without compromising your mental health.1,3
I didn't notice these things were related to a condition that was treatable. I just noticed I started having difficulty with simple tasks like cutting or buttoning, zippering, counting change.
Now Playing:
How to diagnose tardive dyskinesia (TD)
Hear professionals discuss diagnosing tardive dyskinesia and treatment.
Video Transcript:
AUSTEDO® XR (deutetrabenazine) extended-release tablets and AUSTEDO® (deutetrabenazine) tablets are prescription medicines that are used to treat adults with movements in the face, tongue, or other body parts that cannot be controlled (tardive dyskinesia).
IMPORTANT SAFETY INFORMATION
AUSTEDO XR and AUSTEDO can cause serious side effects in people with Huntington’s disease, including: depression, suicidal thoughts, or suicidal actions. Do not start taking AUSTEDO XR or AUSTEDO if you are depressed (have untreated depression or depression that is not well controlled by medicine) or have suicidal thoughts. Pay close attention to any changes, especially sudden changes, in mood, behaviors, thoughts or feelings. This is especially important when AUSTEDO XR or AUSTEDO is started and when the dose is changed. Call your healthcare provider right away if you become depressed, have unusual changes in mood or behavior, or have thoughts of suicide.
Individual results may vary.
Please see the Important Safety Information at the end of this video.
“When diagnosing the patient with Tardive Dyskinesia, we’ll do a history and physical.”
… we’ll do a past medical history, do you have any history of, you know, major depression, bipolar disorder, gastroparesis or, you know, diabetes, and then what medications have you been put on? Have you been put on any medicines that block dopamine? “We look at how many years they’ve been exposed to anti-psychotics. We look at how old they are, what was their base-line psychiatric illness? All of the factors which would be risk factors for developing tardive dyskinesia, we gauge that.” “… many times they’ll say that they’re restless. They can’t sit still. They just don’t feel comfortable in their skin. They’re always moving around. When they’re trying to fall asleep, they’re always sort of going back and forth. Their legs are always moving. Uh, and so they just sort of feel restless. Um, and so many times the family members will just say, “Gosh, they’re just kind of twitchy all the time. You know? They’re always twitching and, and jerking and that sort of thing.”
“… primarily it affects the face and the mouth area, but can affect, any muscle in your body. Primarily, uh, your upper extremities like your hands. Um, it can affect your lower extremities like your feet, and it can affect your torso and up into your neck.” “Once we’ve got a good solid history, which is pointing towards tardive dyskinesia, we do a physical exam. And many practitioners simply examine the patient to look for abnormal movements. But there are also scales available, which are validated for us to gauge for the severity of tardive dyskinesia.”
“…I examine the patient using the abnormal involuntary movement scale, uh, where I rate them as a series of seven areas that I look at and rate them from zero being no movement, to four being very severe movement. And then I total that up, and that gives the score.”
“…Somebody might have minimal or mild symptoms according to a scale like the abnormal involuntary movements scale…but that could be highly disabling for that individual. If it’s affecting their interpersonal life, their occupation, their domestic setting, it’s oftentimes going to evoke embarrassment and avoidance.”
“Now what is important for patients to understand is, it does not matter to your provider what the final tally score is. What matters is, how disabling it is for the patient.”
“…when people’s quality of life goes down, it’s like you and I, we want as solid of an experience of life as we possibly get. And I believe in the golden rule. When you place yourself in someone else’s shoes and you see what’s happening with them based on the fact that they have a clinical diagnosis of something, it’s important to let them know that they can do something about that.”
“We know that from both statistics and from surveys, that it can be … physically … disabling. So why would you leave a disorder as such untreated, which can potentially impact the person in multiple ways.There’s a great degree of heterogeneity, which means variability among people, but it’s kind of nice knowing that we have agents that can help us with a really bad problem that we’ve lived with for many years without really having a solution for it. Before, uh, we would treat patients with antipsychotics and we’d say that you have the possibility of having involuntary movements as a- a part of this treatment. And in the past, we would always say, well we have to just live with it. .... Today, we don’t have to make that trade off. We can treat the involuntary movements, or the tardive dyskinesia, should they arise.”
“… when someone suspects that they’ve got a problem, the best move is always seeking out one’s doctor, if that doctor knows what they’re doing, even if they don’t know what’s going on, they’re gonna seek out a second opinion or seek out a movement disorder specialist or somebody that they know clearly feels comfortable diagnosing and treating individuals with involuntary movements. So I’d say that’s a first step, seeking out the appropriate attention and help and then maybe being led down the path of finding the right medicine for them.”
APPROVED USE
AUSTEDO® XR (deutetrabenazine) extended-release tablets and AUSTEDO® (deutetrabenazine) tablets are prescription medicines that are used to treat:
the involuntary movements (chorea) of Huntington’s disease. AUSTEDO XR and AUSTEDO do not cure the cause of the involuntary movements, and it does not treat other symptoms of Huntington’s disease, such as problems with thinking or emotions.
movements in the face, tongue, or other body parts that cannot be controlled (tardive dyskinesia).
It is not known if AUSTEDO XR and AUSTEDO are safe and effective in children.
IMPORTANT SAFETY INFORMATION
AUSTEDO XR and AUSTEDO can cause serious side effects in people with Huntington’s disease, including: depression, suicidal thoughts, or suicidal actions. Do not start taking AUSTEDO XR or AUSTEDO if you are depressed (have untreated depression or depression that is not well controlled by medicine) or have suicidal thoughts. Pay close attention to any changes, especially sudden changes, in mood, behaviors, thoughts or feelings. This is especially important when AUSTEDO XR or AUSTEDO is started and when the dose is changed. Call your healthcare provider right away if you become depressed, have unusual changes in mood or behavior, or have thoughts of suicide.
Do not take AUSTEDO XR or AUSTEDO if you:
have Huntington’s disease and are depressed or have thoughts of suicide.
have liver problems.
are taking reserpine. Do not take medicines that contain reserpine with AUSTEDO XR or AUSTEDO. If your healthcare provider plans to switch you from taking reserpine to AUSTEDO XR or AUSTEDO, you must wait at least 20 days after your last dose of reserpine before you start taking AUSTEDO XR or AUSTEDO.
are taking a monoamine oxidase inhibitor (MAOI) medicine. Do not take an MAOI within 14 days after you stop taking AUSTEDO XR or AUSTEDO. Do not start AUSTEDO XR or AUSTEDO if you stopped taking an MAOI in the last 14 days. Ask your healthcare provider or pharmacist if you are not sure.
are taking tetrabenazine. If your healthcare provider plans to switch you from tetrabenazine to AUSTEDO XR or AUSTEDO, take your first dose of AUSTEDO XR or AUSTEDO on the day after your last dose of tetrabenazine.
are taking valbenazine.
Other possible serious side effects include:
Irregular heartbeat (QT prolongation). AUSTEDO XR and AUSTEDO increases your chance of having certain changes in the electrical activity in your heart. These changes can lead to a dangerous abnormal heartbeat. Taking AUSTEDO XR or AUSTEDO with certain medicines may increase this chance.
Neuroleptic Malignant Syndrome. Call your healthcare provider right away and go to the nearest emergency room if you develop these signs and symptoms that do not have another obvious cause: high fever, stiff muscles, problems thinking, very fast or uneven heartbeat, or increased sweating.
Restlessness. You may get a condition where you feel a strong urge to move. This is called akathisia.
Parkinsonism. Symptoms include: slight shaking, body stiffness, trouble moving, trouble keeping your balance, or falls.
Sleepiness (sedation) is a common side effect of AUSTEDO XR and AUSTEDO. While taking AUSTEDO XR or AUSTEDO, do not drive a car or operate dangerous machinery until you know how AUSTEDO XR or AUSTEDO affects you. Drinking alcohol and taking other drugs that may also cause sleepiness while you are taking AUSTEDO XR or AUSTEDO may increase any sleepiness caused by AUSTEDO XR and AUSTEDO.
The most common side effects of AUSTEDO in people with Huntington’s disease include sleepiness (sedation), diarrhea, tiredness, and dry mouth.
The most common side effects of AUSTEDO in people with tardive dyskinesia include inflammation of the nose and throat (nasopharyngitis) and problems sleeping (insomnia).
The most common side effects of AUSTEDO XR are expected to be similar to AUSTEDO in people with Huntington’s disease or tardive dyskinesia.
These are not all the possible side effects of AUSTEDO XR or AUSTEDO. Call your doctor for medical advice about side effects. You are encouraged to report side effects of prescription drugs to the FDA. Visit www.fda.gov/medwatch or call 1-800-FDA-1088.
Please read the Medication Guide available at AUSTEDO.com, or by calling 1-800-887-8100.
With Teva Shared Solutions®, you have
options for
starting AUSTEDO XR. Sign up
and get support.
Whether you’re ready to talk to your doctor about
involuntary movements or still trying to figure out what to say, we have
an Appointment Preparation Guide to help you
start the conversation.
The information on this site is intended for healthcare professionals in the United States. Are you a healthcare professional in the United States?
References:
1. AUSTEDO® XR (deutetrabenazine) extended-release tablets/AUSTEDO® tablets current Prescribing Information. Parsippany, NJ: Teva Neuroscience, Inc.
2. Anderson KE, Stamler D, Davis MD, et al. Deutetrabenazine for treatment of involuntary movements in patients with tardive dyskinesia (AIM-TD): a double-blind, randomised, placebo-controlled, phase 3 trial. Lancet Psychiatry. 2017;4(8):595-604.
3. Data on file. Parsippany, NJ: Teva Neuroscience, Inc.
4. Fernandez HH, Stamler D, Davis MD, et al. Long-term safety and efficacy of deutetrabenazine for the treatment of tardive dyskinesia. J Neurol Neurosurg Psychiatry. 2019;90(12):1317-1323.
5. Jackson R, Brams MN, Citrome L, et al. Assessment of the impact of tardive dyskinesia in clinical practice: consensus panel recommendations. Neuropsychiatr Dis Treat. 2021;17:1589-1597.
6. Jain R, Correll CU. Tardive dyskinesia: recognition, patient assessment, and differential diagnosis. J Clin Psychiatry. 2018;79(2):nu17034ah1c.
7. Warikoo N, Schwartz TL, Citrome L. Tardive dyskinesia. In: Schwartz TL, Megna J, Topel ME, eds. Antipsychotic Drugs. Nova Science Publishers, Inc.; 2013:235-258.
8. Waln O, Jankovic J. An update on tardive dyskinesia: from phenomenology to treatment. Tremor Other Hyperkinet Mov (N Y). 2013;3:tre-03-161-4138-1.
9. Tardive dyskinesia. National Alliance on Mental Illness. Accessed January 24, 2024. https://www.nami.org/About-Mental-Illness/Treatments/Mental-Health-Medications/Tardive-Dyskinesia
10. Bergland C. Is tardive dyskinesia reversible? Verywell Health. March 8, 2022. Accessed January 24, 2024. https://www.verywellhealth.com/is-tardive-dyskinesia-reversible-5217232#


